Designated Marketing Approval Holder
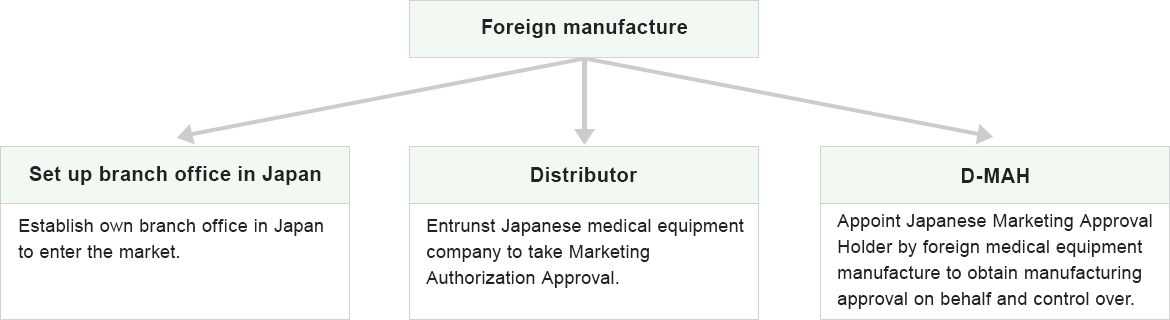
Foreign companies, who are trying to get an approval or certification for their product in Japan, can obtain Approval / Certification directly (Foreign Restrictive Approval.)
However it is required to designate a marketing approval holder (D-MAH) in Japan and only the marketing approval holder is able to “marketing” the imported medical device) (Pharmaceutical Affairs Law Article 19-2).
The company who obtained this approval is called “Foreign restrictive approval holder” and the Designated Marketing Approval Holder is called “D-MAH".
D-MAH has to obtaining the license according to the classification of the product to be handled.
How to launch Foreign Medical Equipment Manufacture business in Japan
Merits / Demerits
| Set up own branch | Distributor | D-MAH | |
|---|---|---|---|
| Incorporation expenses | Require huge budget | No cost | No cost |
| Manpower expenses | Local employment fee paid for by the branch | No cost | No cost |
| Confidential matter | Protected | Release product's classified information | Protected |
| Sales profit | Decline net profit margin by runing cost | Decline net profit by at cost price | Ensured highest net profit margin |
| Running cost (excluding manpower expenses) |
Branch office rent fee, and other overhead expenses | No cost | Reasonable handling and import charges to level with sales profit |
| Regulation | Paid for by the branch | Paid by distributor | Absorb the labor charges As initial cost |
- Colored points are demerits for overseas manufacture
- Otherwise D-MAH practice usage will lead benefit for overseas manufacture
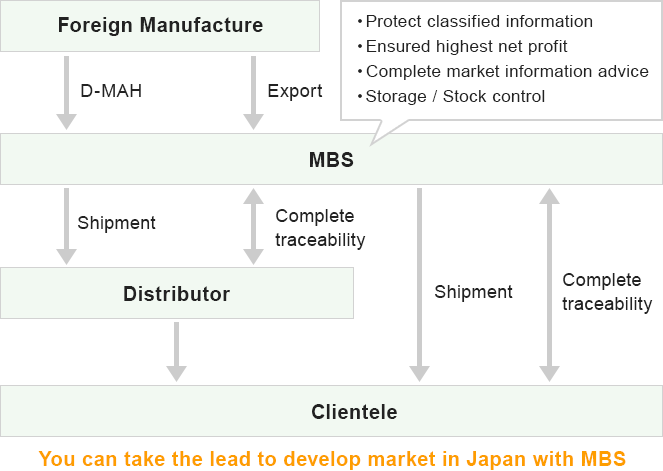
You will be able to manage your business initiative and develop market in japan with highest net profit.
MBS Service Feature
Designate Marketing Approval Holder (D-MAH) required to obtain Foreign Restricted Approval.
MBS obtained following licenses
- No.1 type license for marketing business of medical devices
(capable to handle class I, II, III, IV medical devices) - Marketing Approval of Medical Equipment/packaging ·labeling ·storage ·QA/QC
(import medical devices, storage, management, any labor work such as labeling and distribution where we manage warehouse in Tokyo-bay) - Specially controlled medical devices marketing
(capable of direct sales to the final clientele)
Obtained other licenses
- License for marketing business of cosmetics
- License for manufacturing cosmetics / packaging ·labeling ·storage ·QA/QC
- License for manufacturing medical drugs / in vitro diagnostic reagent ·packaging ·labeling ·storage ·QA/QC
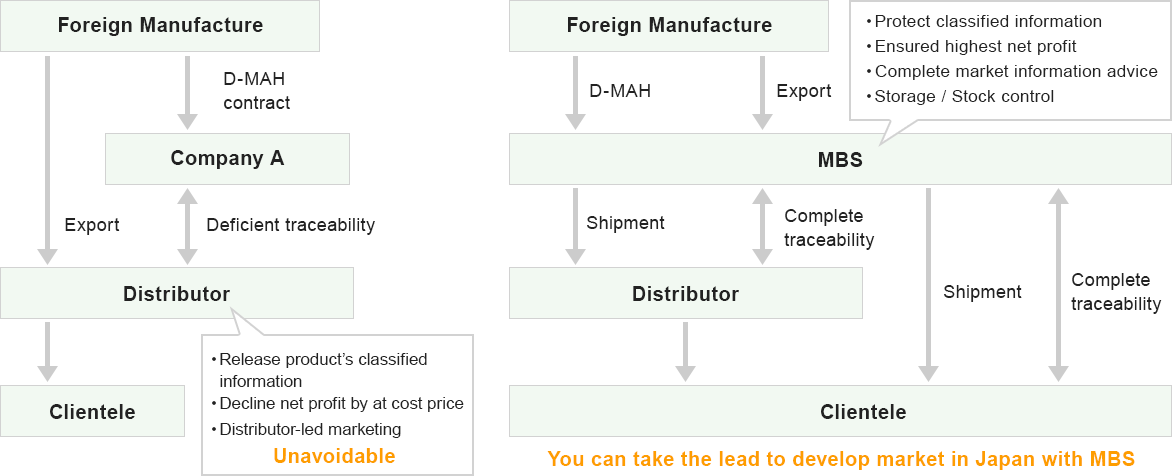
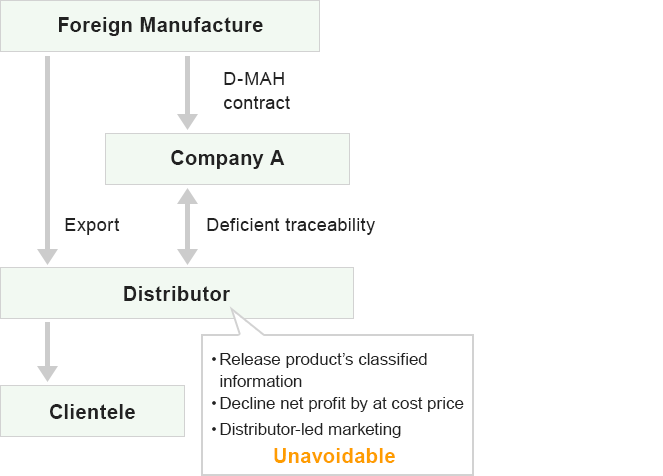
Comparison with other firm